
Becky Lamis, PharmD, FISMP
Manager, Med Safety Board (MSB)
While 503B outsourcing facilities must meet current good manufacturing practice requirements, they are not subjected to the same labeling and packaging safety standards and review process as recommended or required for commercial manufacturers by the US Food and Drug Administration (FDA). The resulting effect of this lack of similar labeling and packaging safety standards for 503B outsourcing facilities has been borne out through numerous reports submitted to Med Safety Board’s parent organization, the Institute for Safe Medication Practices (ISMP).
Common labeling and packaging safety issues involving 503B outsourcing facility products that have been reported through the ISMP National Medication Errors Reporting Program include:
Prefilled syringe labels — per mL amount vs. per total volume
Prefilled syringe labels with the primary display of strength expressed as the per mL amount, instead of the amount per total volume, leading to mistakes in which the per mL strength was interpreted as the total amount in the syringe. The following example is described by ISMP in an article entitled “Inpatient Ketamine Overdose with 503B Compounded Syringe“:

“A patient was supposed to receive ketamine 10 mg as needed for pain. The patient’s nurse removed a 503B outsourced prefilled ketamine syringe from an automated dispensing cabinet (ADC) to administer a dose. The ketamine was packaged in a 5 mL syringe prominently labeled as ‘Ketamine, 10 mg/mL.’ Unfortunately, the nurse did not recognize the total volume in the syringe (5 mL) and the entire 50 mg amount was administered. Because of the 5-fold overdose, the patient became somnolent but, fortunately, recovered.” See Figure 1.
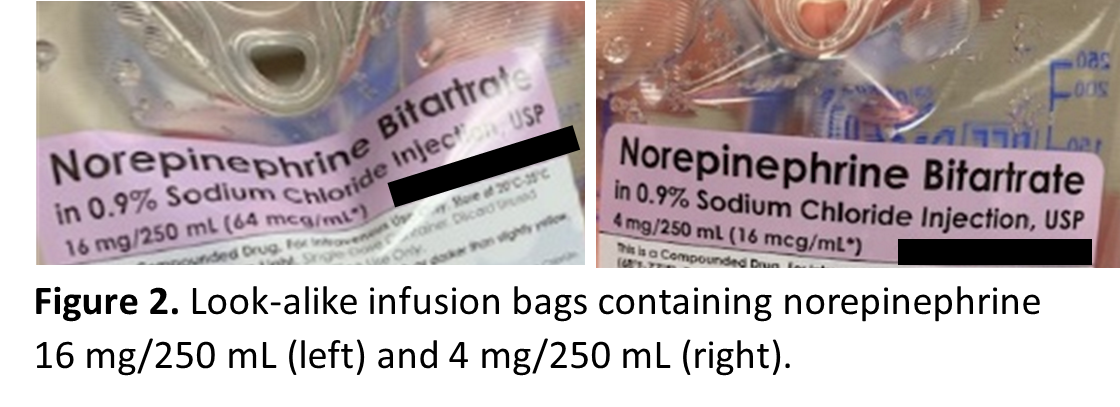
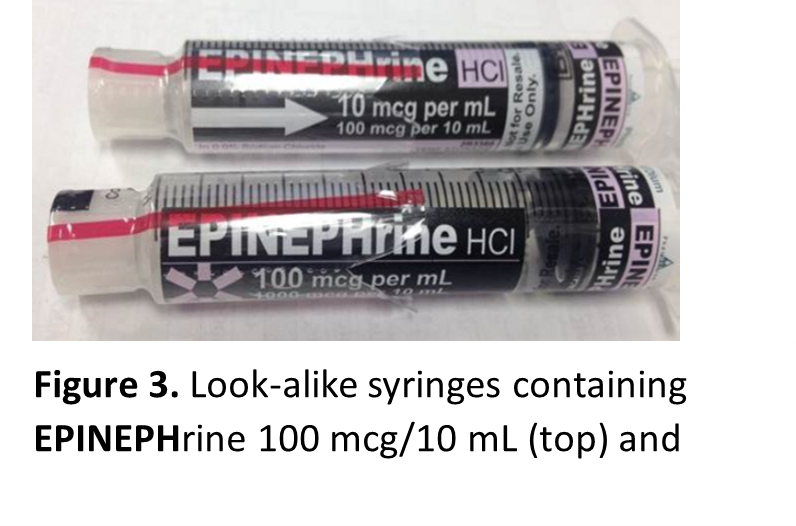
Look-alike infusion bags, prefilled syringes, and vials
Look-alike infusion bags (Figure 2) and prefilled syringes (Figure 3), particularly when using the ASTM International standard color for user-applied labels in anesthesiology (which has since been withdrawn) for all drugs within the same drug class supplied by the outsourcing facility. These similar-looking labels have resulted in mix-ups between different drugs or different concentrations of the same medication.
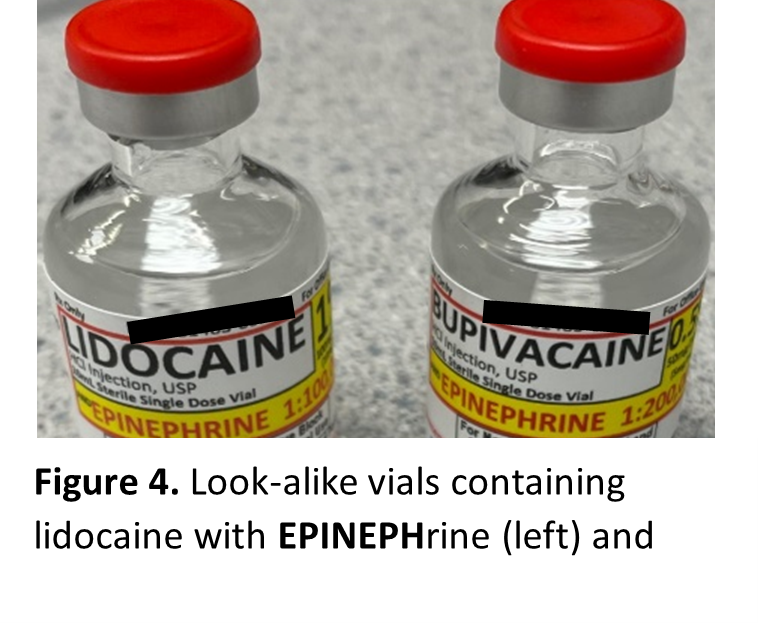
Similar-looking vials have also been reported, as demonstrated by the following event:
“An operating room (OR) ADC pocket intended for bupivacaine 0.5% with EPINEPHrine 1:200,000 was inadvertently also stocked with vials containing lidocaine 1% with EPINEPHrine 1:100,000.” See Figure 4.
Product Container Confusion
Products supplied in containers typically used for products given by a different route of administration, such as preparing medications that require dilution (e.g., potassium chloride concentrate) in prefilled syringes, risking inadvertent direct intravenous (IV) injection, and topical medications in parenteral syringes with a Luer connector that could accidentally be given IV (see Figure 5).

Resulting action
Based on these reported issues, in 2018, ISMP called upon 503B outsourcing facilities or compounders to follow the same safety standards as commercial manufacturers for labeling in an article entitled “FDA Guidance Needed to Assure Safe Labeling Practices by 503A and 503B Compounders.” And, due to these persistent reports and consequent concern regarding patient harm, ISMP even identified unsafe labeling of prefilled syringes and infusions by 503B compounders as one of its Top 10 Medication Errors and Hazards based on reports submitted by healthcare practitioners in 2019.
Since the publication of ISMP’s call to action for 503B outsourcing facilities to improve the safety of their product labeling, some compounders have taken significant steps to change their labels, particularly to ensure that the amount per container is the prominent expression of strength on the label, and with some facilities even going the extra mile by implementing innovative strategies to further enhance the safety of their medication labels. However, issues involving 503 outsourcing facility products continue to be reported to ISMP, indicating that safety improvements are still needed, and with the ever-growing list of drug shortages and compounders stepping in to fulfill some of these product needs, 503B outsourcing facilities should work towards meeting FDA’s standards for safe medication labeling and packaging as a high priority.
Until additional oversight by the FDA is required, 503B outsourcing facilities should voluntarily follow some of the same labeling and packaging safety requirements and guidance from FDA as commercial manufacturers. This includes reviewing FDA’s guidance for industry on Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors and Safety Considerations for Product Design to Minimize Medication Errors and applying relevant labeling and packaging recommendations to their portfolio of products.
Given its affiliation with ISMP, and its bench of experienced and licensed strategic resources, MSB can support you in optimizing patient safety and minimizing labeling errors that can lead to negative patient safety outcomes. We help 503B outsourcers in improving the safety of their products’ labeling and packaging and following pertinent FDA guidance through various consulting services, including labeling and packaging design and review, risk assessments, human factors evaluations, focus groups, and error analysis.
Contact Med Safety Board to learn more and to speak with our experts,
or call (267) 725-0063 for a Complimentary Discovery Call to discuss your potential risks and the solutions that we offer.